NextGen 2024: Weaning meds when disease is controlled Session Part 1
 AI Summary
AI Summary
Key Insights
- The Utrecht protocol uses rIL-1RA as a first-line treatment for new-onset Systemic Juvenile Idiopathic Arthritis (SJIA) and is effective in a national multicenter setting.
- Adding IL-18 as a biomarker (with a cutoff value of 1200 pg/mL) improves the success of tapering rIL-1RA in SJIA patients achieving Clinical Inactive Disease (CID) at 3 months with rIL-1RA monotherapy.
- Early tapering and stopping reduces the treatment duration and amount of injections.
- Biological treatments targeting IL1 (anakinra, canakinumab) and IL6 (tocilizumab) pathways show improved prognosis and reduce the need for steroids, though potential side effects include osteoporosis, slowed growth, and increased risk of infections.




NextGen 2024: Weaning meds when disease is controlled Session Part 1
- 1. Biomarker-Guided Treatment-And-Stop-Strategy for Recombinant IL-1Receptor Antagonist (anakinra) in Systemic Juvenile Idiopathic Arthritis Bas Vastert, MD, PhD Head of the department of pediatric rheumatology and immunology, Wilhelmina Children’s Hospital Center for Translational Immunology, UMC Utrecht
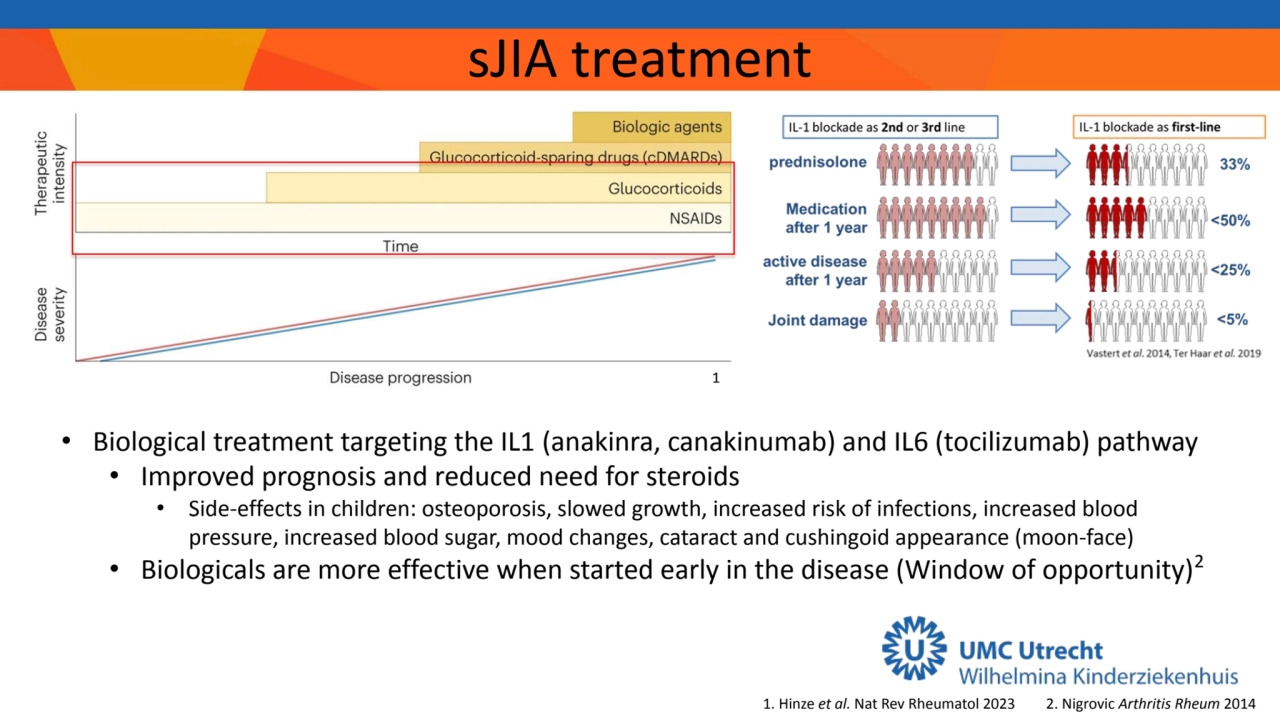
- 2. sJIA treatment 2. Nigrovic Arthritis Rheum 2014 • Biological treatment targeting the IL1 (anakinra, canakinumab) and IL6 (tocilizumab) pathway • Improved prognosis and reduced need for steroids • Side-effects in children: osteoporosis, slowed growth, increased risk of infections, increased blood pressure, increased blood sugar, mood changes, cataract and cushingoid appearance (moon-face) • Biologicals are more effective when started early in the disease (Window of opportunity)2 1. Hinze et al. Nat Rev Rheumatol 2023 1
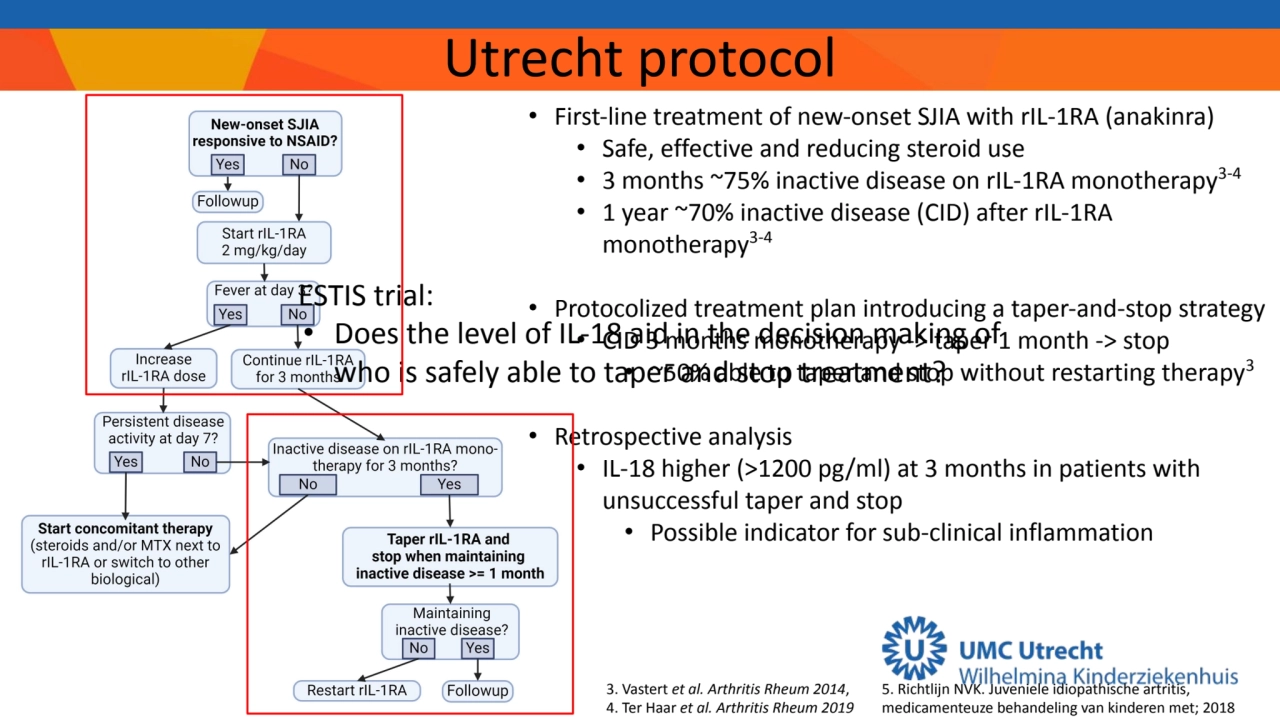
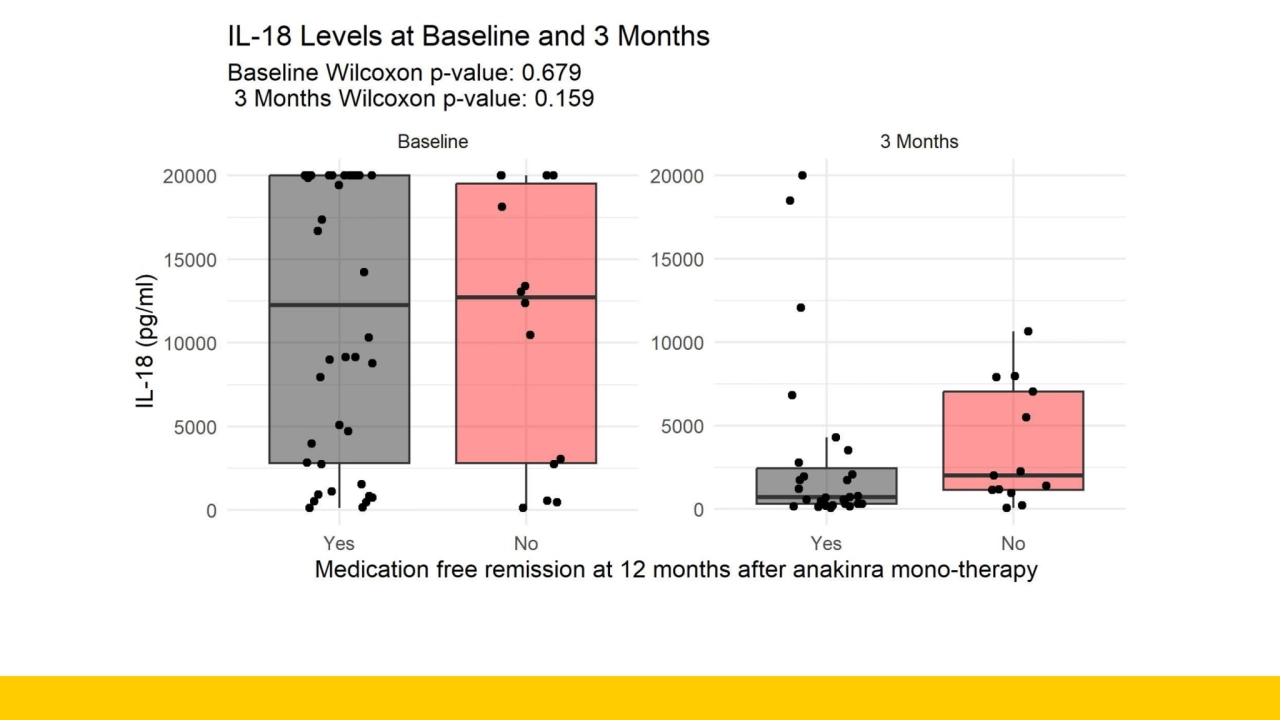
- 3. 3. Vastert et al. Arthritis Rheum 2014, 4. Ter Haar et al. Arthritis Rheum 2019 Utrecht protocol • First-line treatment of new-onset SJIA with rIL-1RA (anakinra) • Safe, effective and reducing steroid use • 3 months ~75% inactive disease on rIL-1RA monotherapy3-4 • 1 year ~70% inactive disease (CID) after rIL-1RA monotherapy3-4 • Protocolized treatment plan introducing a taper-and-stop strategy • CID 3 months monotherapy -> taper 1 month -> stop • ~50% able to taper and stop without restarting therapy3 • Retrospective analysis • IL-18 higher (>1200 pg/ml) at 3 months in patients with unsuccessful taper and stop • Possible indicator for sub-clinical inflammation 5. Richtlijn NVK. Juveniele idiopathische artritis, medicamenteuze behandeling van kinderen met; 2018 ESTIS trial: • Does the level of IL-18 aid in the decision making of who is safely able to taper and stop treatment?
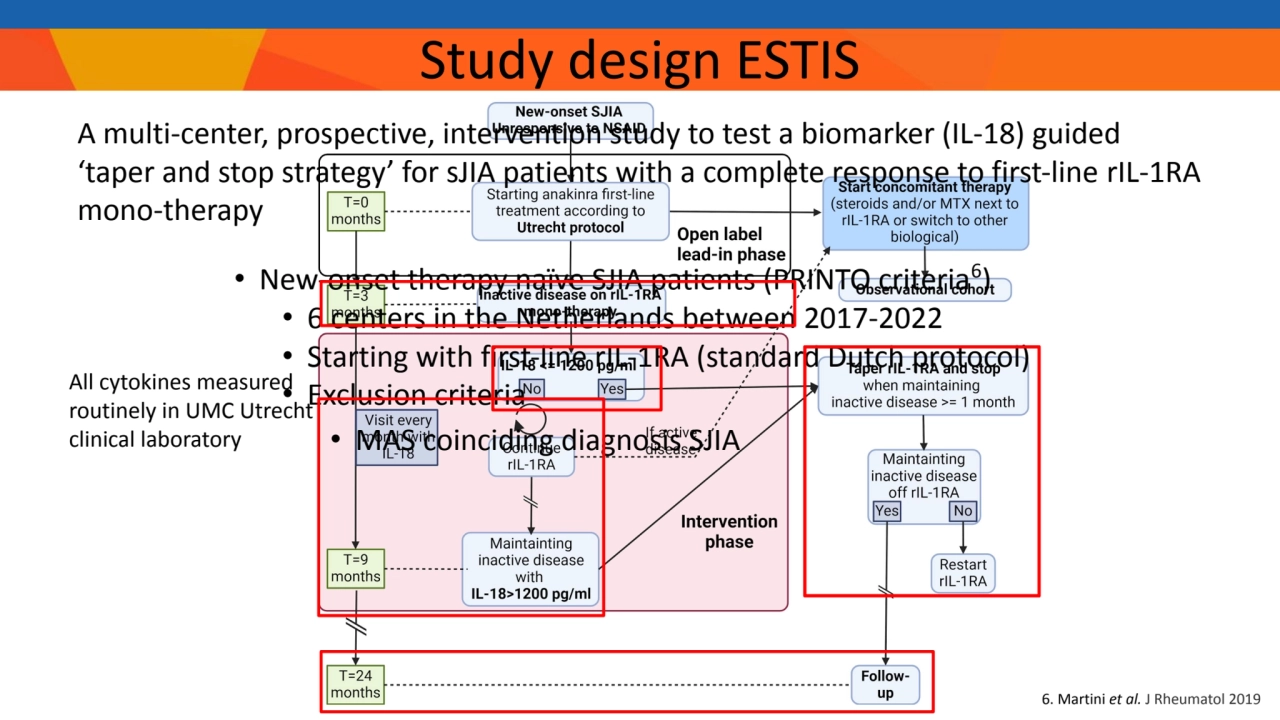
- 4. • New-onset therapy naïve SJIA patients (PRINTO criteria6 ) • 6 centers in the Netherlands between 2017-2022 • Starting with first-line rIL-1RA (standard Dutch protocol) • Exclusion criteria • MAS coinciding diagnosis SJIA 6. Martini et al. J Rheumatol 2019 Study design ESTIS A multi-center, prospective, intervention study to test a biomarker (IL-18) guided ‘taper and stop strategy’ for sJIA patients with a complete response to first-line rIL-1RA mono-therapy All cytokines measured routinely in UMC Utrecht clinical laboratory
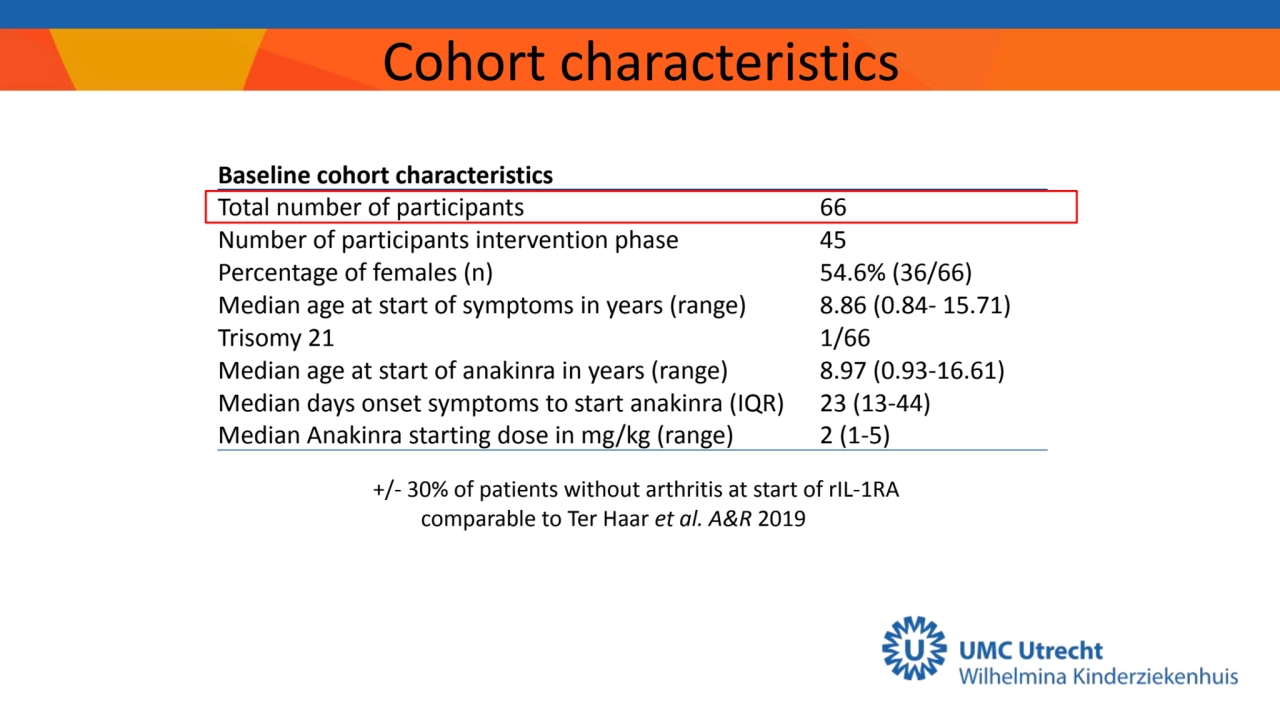
- 5. Baseline cohort characteristics Total number of participants 66 Number of participants intervention phase 45 Percentage of females (n) 54.6% (36/66) Median age at start of symptoms in years (range) 8.86 (0.84- 15.71) Trisomy 21 1/66 Median age at start of anakinra in years (range) 8.97 (0.93-16.61) Median days onset symptoms to start anakinra (IQR) 23 (13-44) Median Anakinra starting dose in mg/kg (range) 2 (1-5) Cohort characteristics +/- 30% of patients without arthritis at start of rIL-1RA comparable to Ter Haar et al. A&R 2019
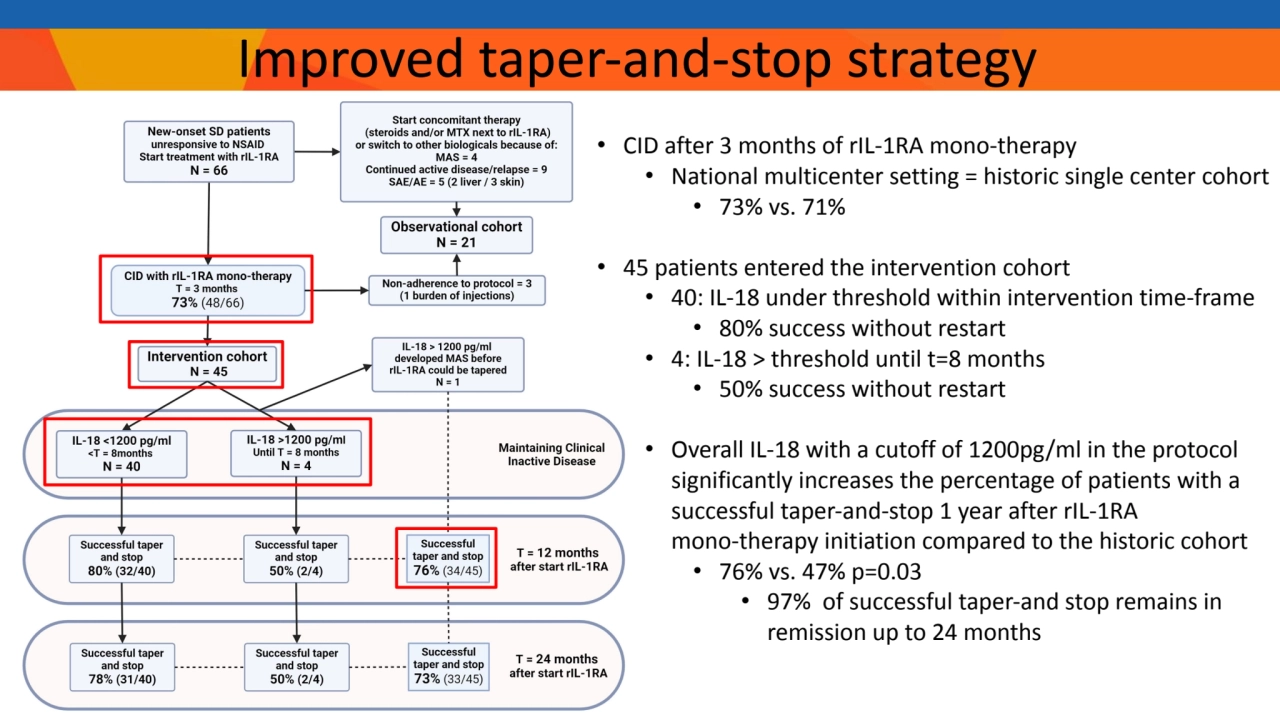
- 6. • CID after 3 months of rIL-1RA mono-therapy • National multicenter setting = historic single center cohort • 73% vs. 71% • 45 patients entered the intervention cohort • 40: IL-18 under threshold within intervention time-frame • 80% success without restart • 4: IL-18 > threshold until t=8 months • 50% success without restart • Overall IL-18 with a cutoff of 1200pg/ml in the protocol significantly increases the percentage of patients with a successful taper-and-stop 1 year after rIL-1RA mono-therapy initiation compared to the historic cohort • 76% vs. 47% p=0.03 • 97% of successful taper-and stop remains in remission up to 24 months Improved taper-and-stop strategy
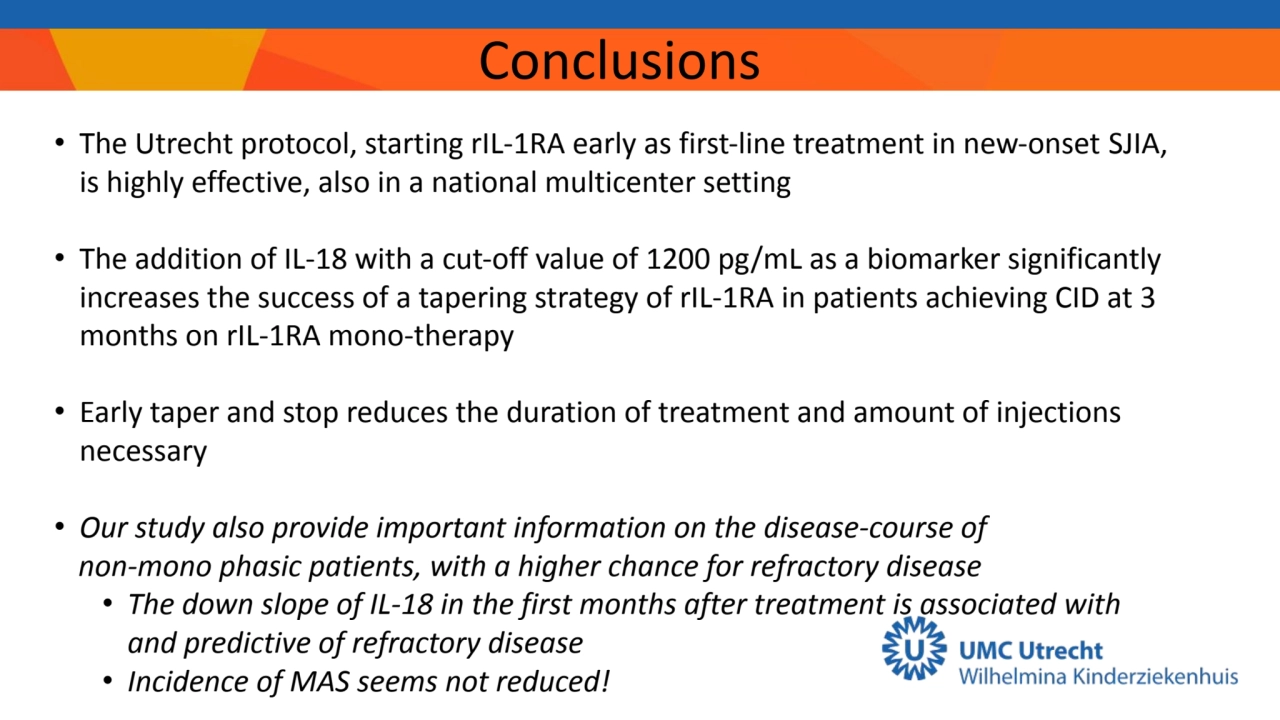
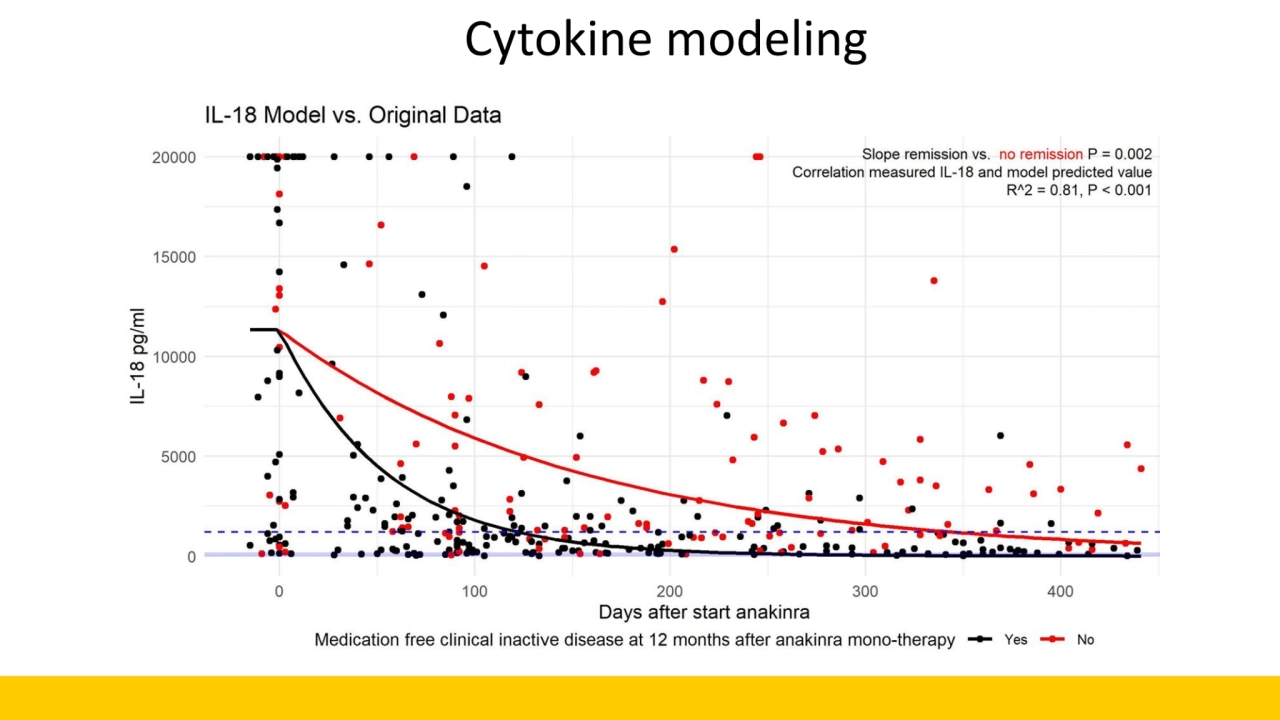
- 7. Conclusions • The Utrecht protocol, starting rIL-1RA early as first-line treatment in new-onset SJIA, is highly effective, also in a national multicenter setting • The addition of IL-18 with a cut-off value of 1200 pg/mL as a biomarker significantly increases the success of a tapering strategy of rIL-1RA in patients achieving CID at 3 months on rIL-1RA mono-therapy • Early taper and stop reduces the duration of treatment and amount of injections necessary • Our study also provide important information on the disease-course of non-mono phasic patients, with a higher chance for refractory disease • The down slope of IL-18 in the first months after treatment is associated with and predictive of refractory disease • Incidence of MAS seems not reduced!
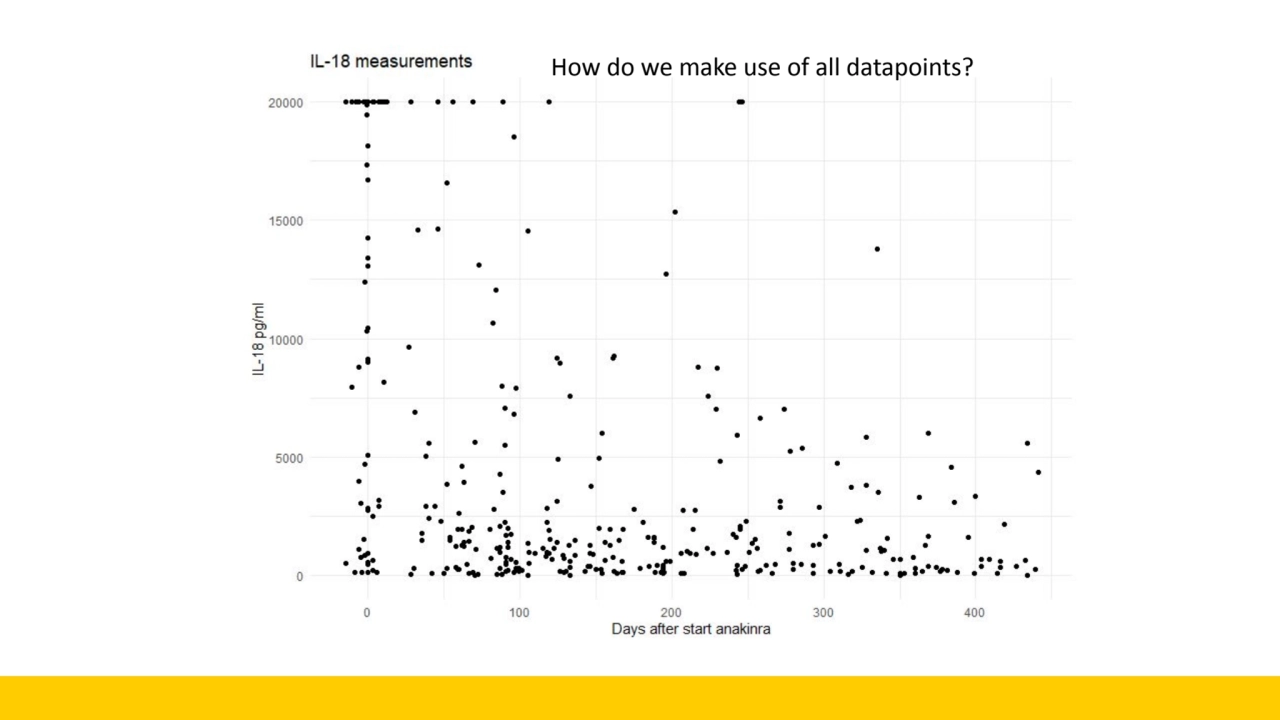
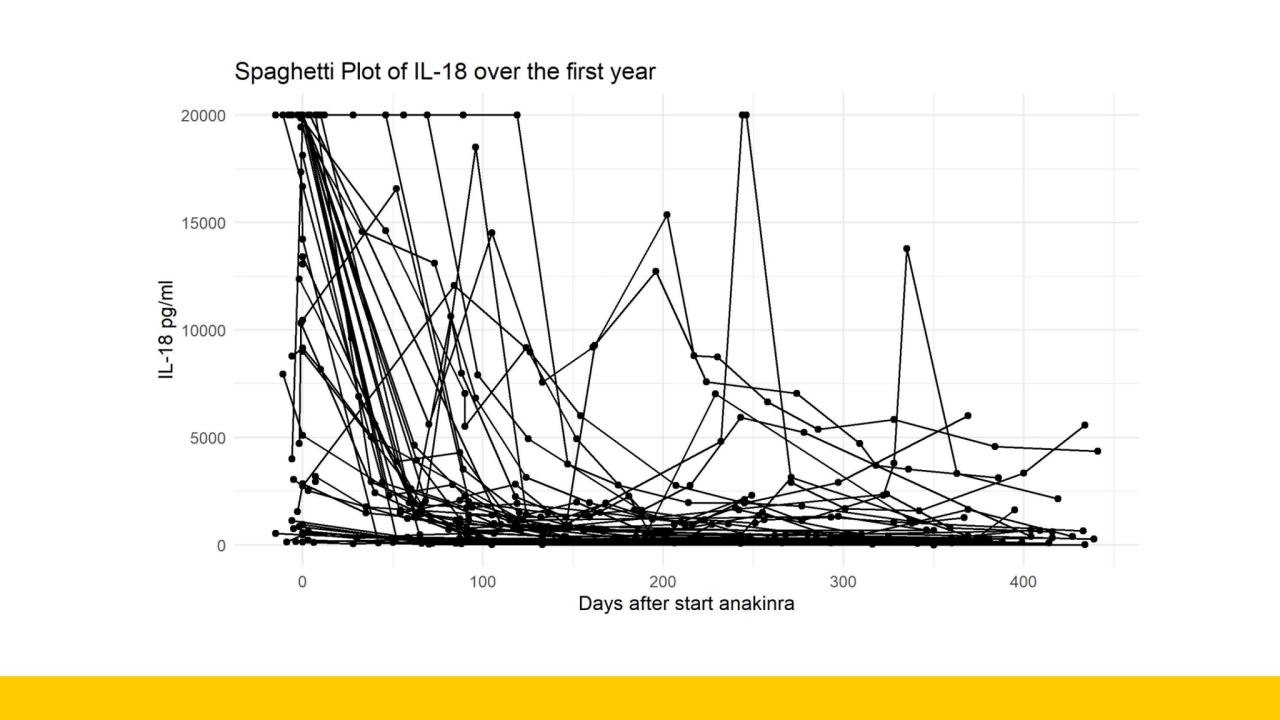
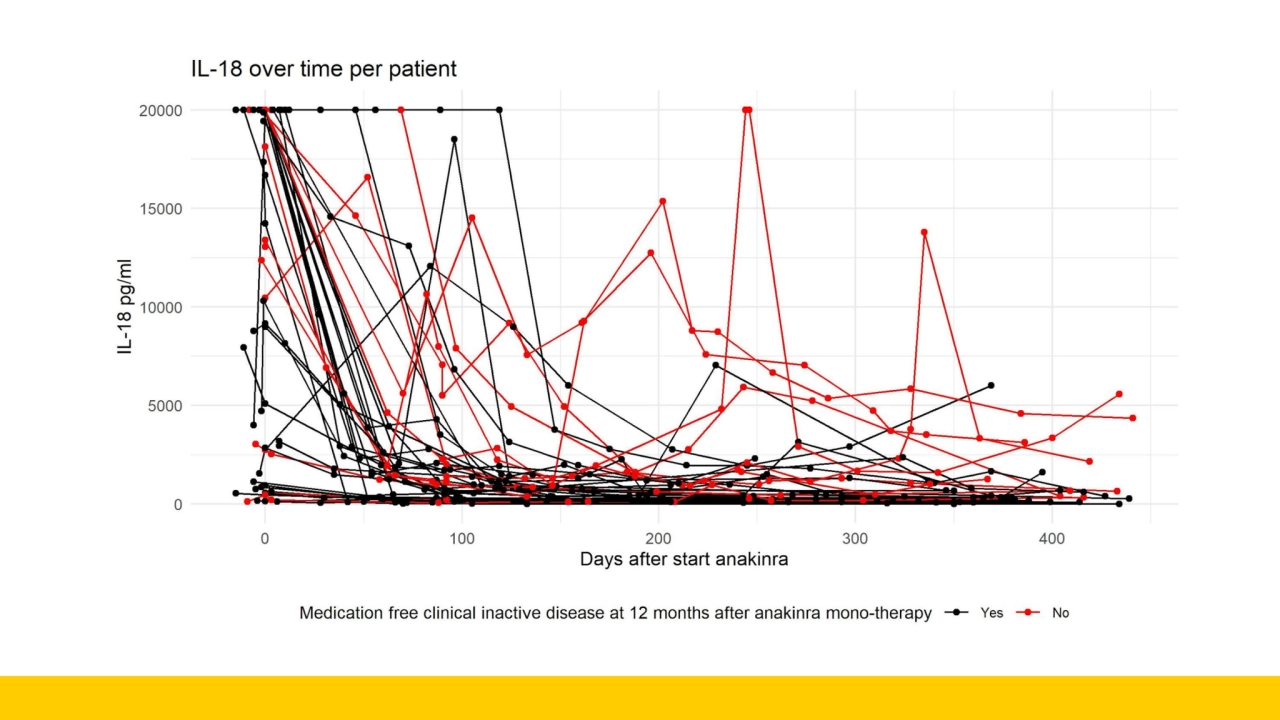
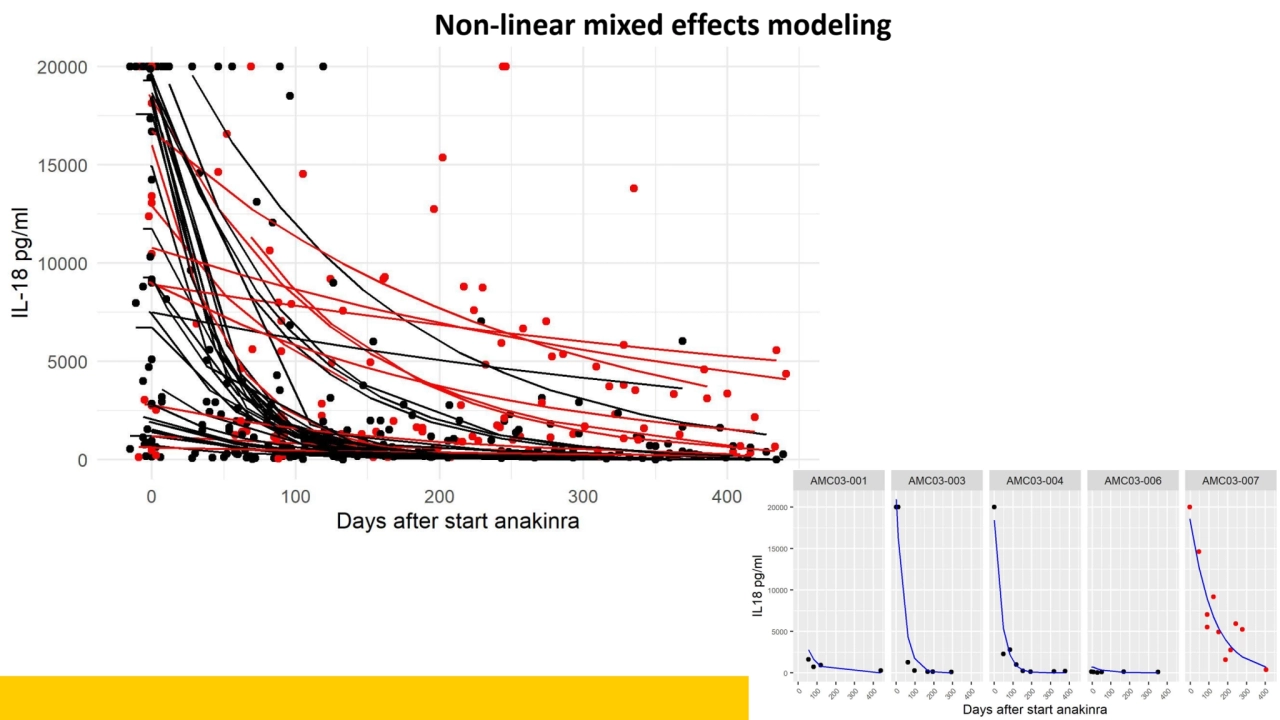
- 8. How do we make use of all datapoints?
- 12. Non-linear mixed effects modeling
- 13. Cytokine modeling
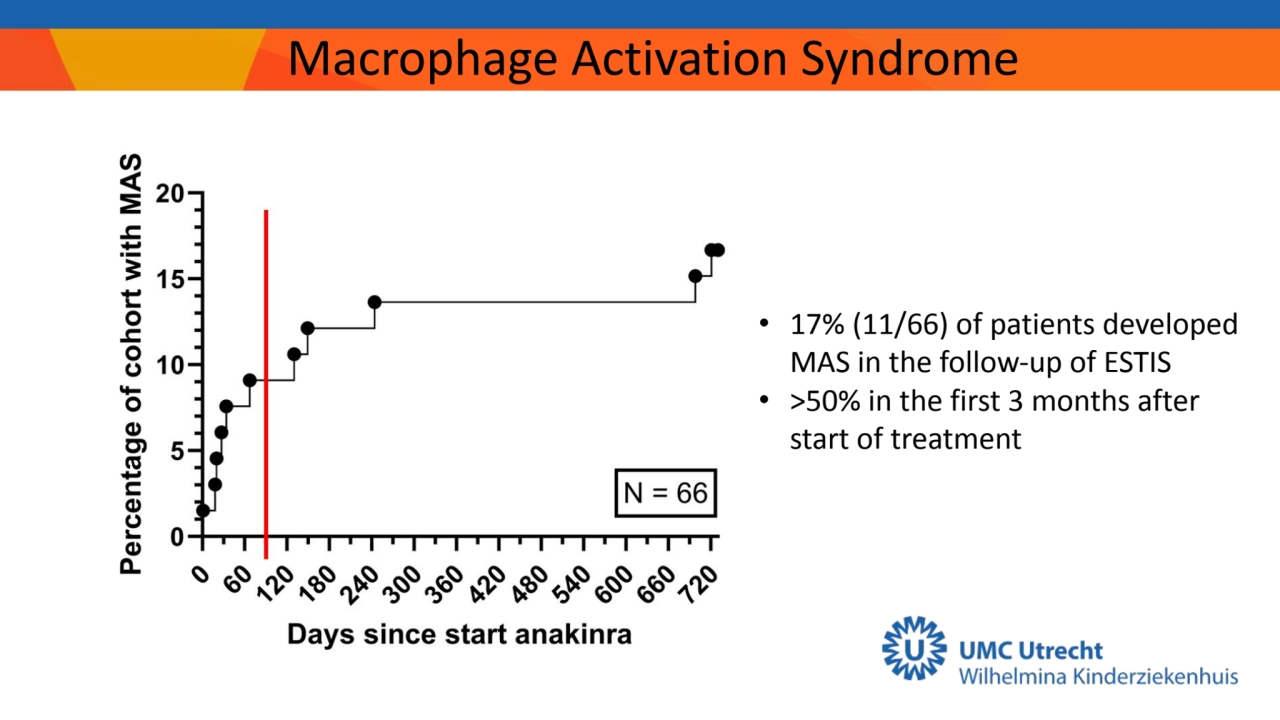
- 14. Macrophage Activation Syndrome • 17% (11/66) of patients developed MAS in the follow-up of ESTIS • >50% in the first 3 months after start of treatment
- 15. Sytze De Roock Gerda Den Engelsman Marc Jansen Johannes Roth Joost Swart Thomas Vogl Petra Hissink Muller Esther Hoppenreijs Ellen Schatorjé Marleen Verkaaik Sylvia Kamphuis Elizabeth Legger Wineke Armbrust Mariken Gruppen Merlijn Van den Berg Dieneke Schonenberg-Meinema Dörte Hamann Acknowledgements Remco Erkens Erika v Nieuwenhove
- 16. Discussion
- 17. Anakinra side-effects: Local reaction at injection site (redness, itching) generally, appear within 2 weeks of therapy, disappear within 4–6 weeks (transient) liver enzyme elevations Potential increased risk of infections (mainly respiratory) Allergic reactions (anaphylaxis) Intervention cohort Observational cohort P Percentage of females (n) 42.2% (19/45) 81% (17/21) 0.007