NextGen 2024: Unmet Needs & Clinical Trial Challenges Session Part 2
 AI Summary
AI Summary
Key Insights
- IL-1 and IL-6 inhibitors are highly effective in 70-80% of Still's disease patients, becoming a standard early treatment.
- Acceptability of experimental drugs and randomization is a key consideration for patients/parents in clinical trials.
- Refractory (D2T) Still's disease presents challenges in clinical trials, including persistent arthritis, relapsing MAS, and acute MAS flares.
- Different criteria apply to classify and assess response in different scenarios of Refractory (D2T) Still's disease
- Clinical trial designs for refractory Still's disease face ethical and practical challenges, particularly for relapsing/smoldering MAS and acute MAS flares.
Loading...

Loading...

Loading...
Loading...
Loading...

Loading...

Loading...
Loading...
Loading...
Loading...

Loading...

NextGen 2024: Unmet Needs & Clinical Trial Challenges Session Part 2
- 1. Clinical Trials in Still´s disease (sJIA/AOSD) Fabrizio de Benedetti Rheumatology, Children Hospital Bambino Gesu’, Rome - Italy
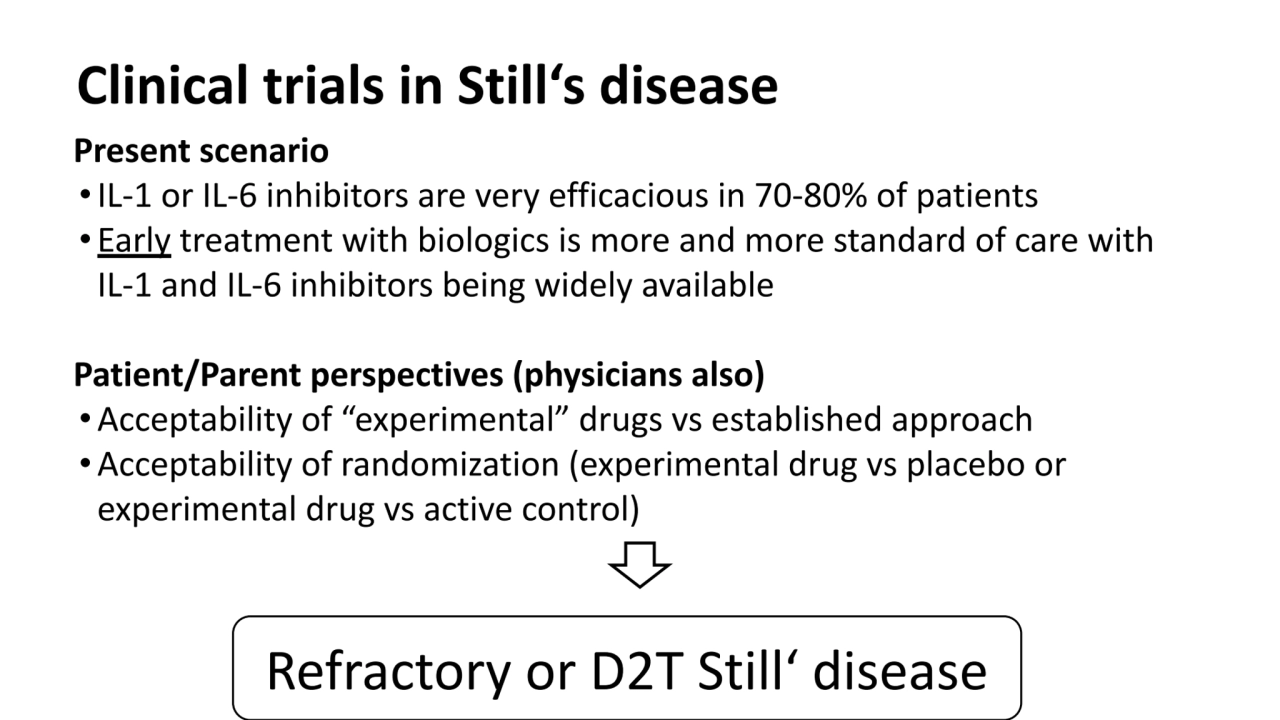
- 2. Clinical trials in Still‘s disease Present scenario •IL-1 or IL-6 inhibitors are very efficacious in 70-80% of patients • Early treatment with biologics is more and more standard of care with IL-1 and IL-6 inhibitors being widely available Patient/Parent perspectives (physicians also) •Acceptability of “experimental” drugs vs established approach •Acceptability of randomization (experimental drug vs placebo or experimental drug vs active control)
- 3. Clinical trials in Still‘s disease Present scenario •IL-1 or IL-6 inhibitors are very efficacious in 70-80% of patients • Early treatment with biologics is more and more standard of care with IL-1 and IL-6 inhibitors being widely available Patient/Parent perspectives (physicians also) •Acceptability of “experimental” drugs vs established approach •Acceptability of randomization (experimental drug vs placebo or experimental drug vs active control) Refractory or D2T Still‘ disease
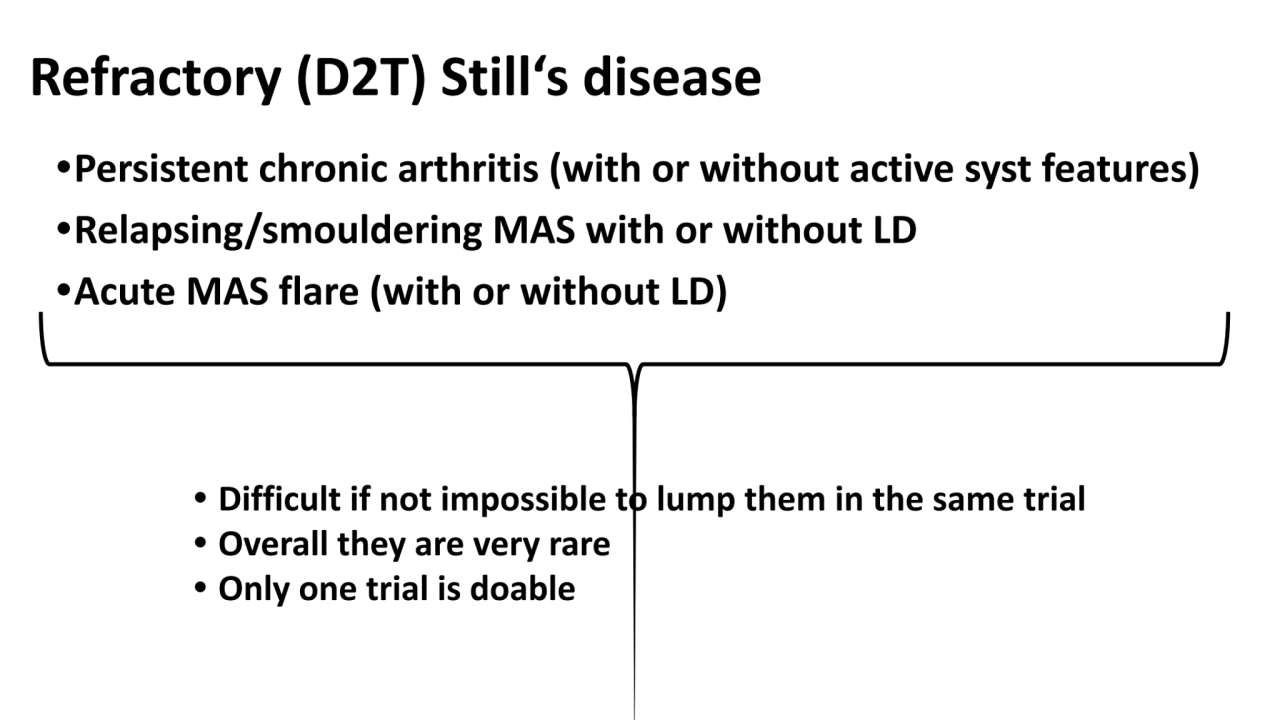
- 4. Refractory (D2T) Still‘s disease •Persistent chronic arthritis (with or without active syst features) •Relapsing/smouldering MAS with or without LD •Acute MAS flare (with or without LD) • Difficult if not impossible to lump them in the same trial • Overall they are very rare • Only one trial is doable
- 5. Refractory (D2T) Still‘s disease 1. persistent chronic arthritis (with or without active systemic features) Classification criteria: OK with ILAR, Yamaguchi, provisional PRINTO Response criteria: • OK with JIA-ACR response + absence of fever, systJADAS (merging adults and children criteria) • Tapering/withdrawal of background treatment (e.g. GC) Eligible for ongoing trials in pcJIA (e.g. Jak inhibitors), only if systemic symptoms are absent • Need new MoA (limited safety data) • 12 and older 🡪 PK modeling for dosing in <12yo • Sample size • Randomization (placebo? Active control?) • Open label • External (historical) controls
- 6. Refractory (D2T) Still‘s disease 1. persistent chronic arthritis (with or without active systemic features) Classification criteria: OK with ILAR, Yamaguchi, provisional PRINTO Response criteria: • OK with JIA-ACR response + absence of fever, systJADAS (merging adults and children criteria • Tapering/withdrawal of background treatment (e.g. GC) • Eligible for ongoing trials in JIA (e.g. Jak inhibitors), only if systemic symptoms are absent • Need new MoA (limited safety data) • 12 and older 🡪 PK modeling for dosing in <12yo • Sample size • Randomization (placebo? Active control?) • Open label • External (historical) controls
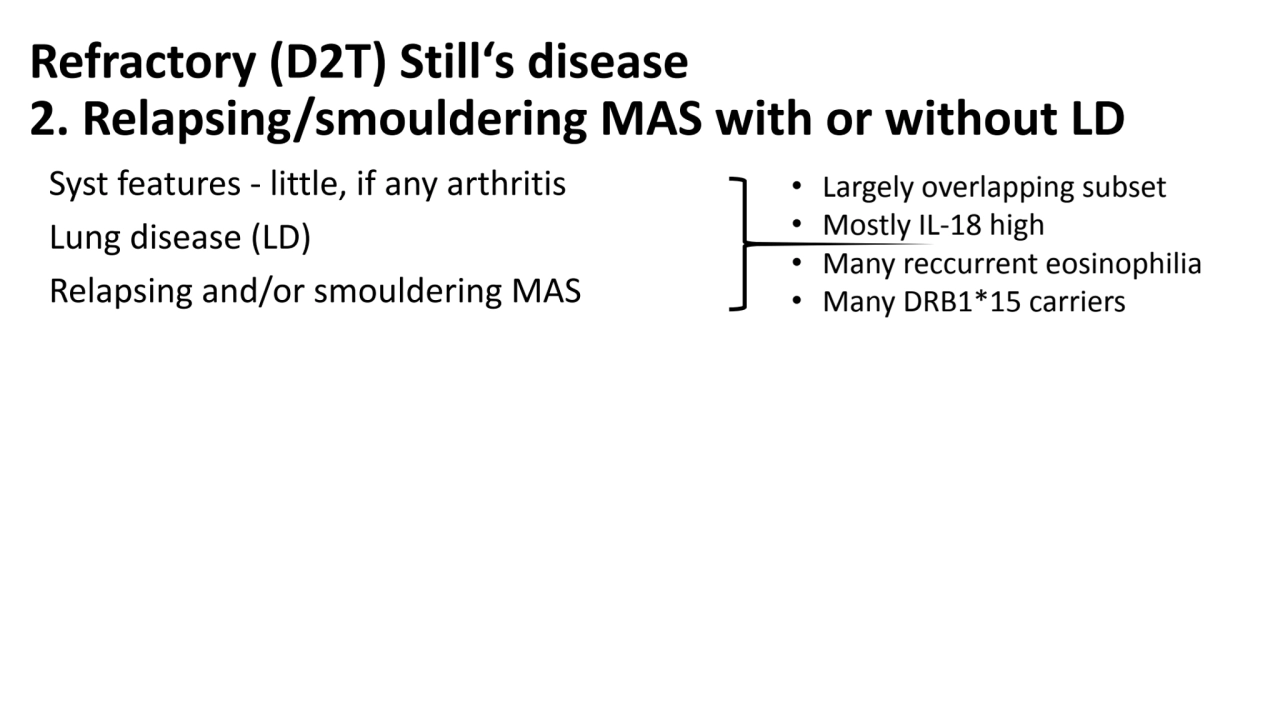
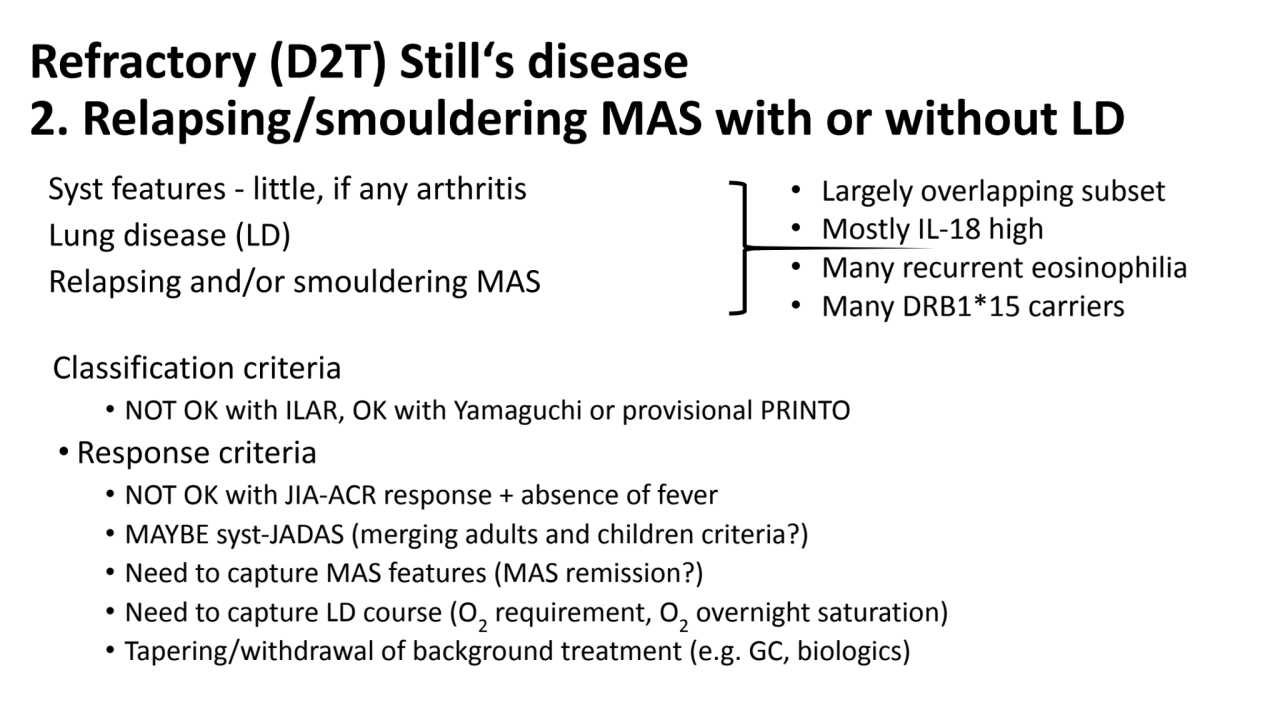
- 7. Refractory (D2T) Still‘s disease 2. Relapsing/smouldering MAS with or without LD Syst features - little, if any arthritis Lung disease (LD) Relapsing and/or smouldering MAS Classification criteria • NOT OK with ILAR, might be OK with Yamaguchi or provisional PRINTO • Response criteria • NOT OK with JIA-ACR response + absence of fever • MAYBE syst-JADAS (merging adults and children criteria) • Tapering/withdrawal of background treatment (e.g. GC, biologics) • Largely overlapping subset • Mostly IL-18 high • Many reccurrent eosinophilia • Many DRB1*15 carriers
- 8. Refractory (D2T) Still‘s disease 2. Relapsing/smouldering MAS with or without LD Syst features - little, if any arthritis Lung disease (LD) Relapsing and/or smouldering MAS Classification criteria • NOT OK with ILAR, OK with Yamaguchi or provisional PRINTO • Response criteria • NOT OK with JIA-ACR response + absence of fever • MAYBE syst-JADAS (merging adults and children criteria?) • Need to capture MAS features (MAS remission?) • Need to capture LD course (O2 requirement, O2 overnight saturation) • Tapering/withdrawal of background treatment (e.g. GC, biologics) • Largely overlapping subset • Mostly IL-18 high • Many recurrent eosinophilia • Many DRB1*15 carriers
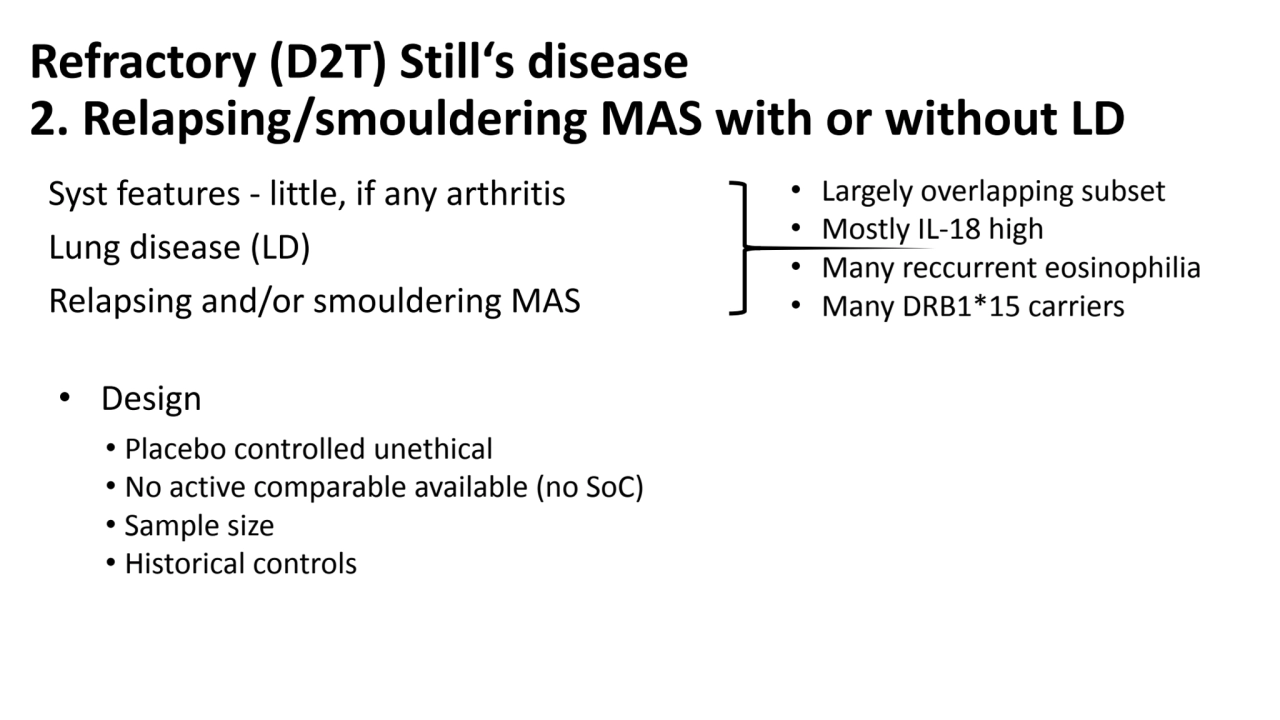
- 9. Refractory (D2T) Still‘s disease 2. Relapsing/smouldering MAS with or without LD Syst features - little, if any arthritis Lung disease (LD) Relapsing and/or smouldering MAS • Design • Placebo controlled unethical • No active comparable available (no SoC) • Sample size • Historical controls • Largely overlapping subset • Mostly IL-18 high • Many reccurrent eosinophilia • Many DRB1*15 carriers
- 10. Refractory (D2T) Still‘s disease 3. Acute MAS flare (with or without LD) Classification criteria • OK with 2016 MAS criteria • NOT OK with ILAR because severe MAS present often at disease onset • OK with Yamaguchi or provisional PRINTO Response criteria • MAS remission as defined in the emapalumab trial -> no formal validation • Tapering/withdrawal of background treatment (e.g. GC, biologics) Design • Placebo controlled unethical • No active comparable available (SoC?) • Background treatment for Still´s disease • Sample size • Historical controls
- 11. Refractory (D2T) Still‘s disease 3. Acute MAS flare (with or without LD) Classification criteria • OK with 2016 MAS criteria • NOT OK with ILAR because severe MAS present often at disease onset • OK with Yamaguchi or provisional PRINTO Response criteria • MAS remission as defined in the emapalumab trial -> no formal validation • Tapering/withdrawal of background treatment (e.g. GC, biologics) Design • Placebo controlled unethical • No active comparable available (SoC?) • Background treatment for Still´s disease • Sample size • Historical controls