NextGen 2024: Weaning meds when disease is controlled Session Part 2
 AI Summary
AI Summary
Key Insights
- The document discusses the weaning therapy in Systemic Juvenile Idiopathic Arthritis (SJIA).
- Patient cases and patterns of SJIA are presented, including recurrent, persistent, and continuous therapy needs.
- The information highlights the importance of following clinical clues, lab results, and genetic/biologic factors in understanding and managing SJIA.
- Gaps in the current approach are identified such as improving genetic test interpretation, understanding epigenetics, and addressing drug efficacy issues.
- Multidisciplinary care, rapid disease control, and shared decision-making are essential aspects of SJIA management.
Loading...

Loading...

Loading...

Loading...

Loading...
Loading...

Loading...
Loading...
Loading...
Loading...
Loading...
Loading...

Loading...

Loading...

Loading...

NextGen 2024: Weaning meds when disease is controlled Session Part 2
- 1. SJIA Weaning Therapy Fatma Dedeoglu, MD Boston Children’s Hospital Harvard Medical School 11/15/2024
- 2. Disclosures UpToDate (Royalties) SOBI (Honorarium)
- 3. PATTERNS Recurrent/Episodic with long off medication Persistent but with medication free periods Persistent with need for continuous therapy 3
- 4. Patient 1 • 18 yo M • Dx Still’s • Very high ESR (130), CRP (25 mg/dl) • High WBC, plt • IL-18 9000 range, CXCL9 nl • Anakinra with wean to QOD in 4 months • Flare in 1 week back to daily anakinra 4
- 5. • 13 yo F Patient 2 • Presented at age 4 • Multiple MAS episodes, evolving into autoimmunity (Sjogren’s) • Anakinra, canakinumab, tocilizumab • Prolonged and intermittent steroids (no use for 2 ½ years) • Tacrolimus • Jakinibs ( ruxolitinib) • Elevated persistent IL-18 during remission • IL-18/ CXCL9: 300000 in 2018 (ferritin: 1600) Ferritin: Normal-300 71000/ 254- in 2019 28000 – in 2020 -> 15000 in 2020 67000/ 3290 in 2021 -> 23000/1742 in 2021; 38000/3267; 42000/1950 in 2022 …… • 2024: MAS flare during switch to jakinib & viral illness 5
- 6. Patient 3 • 22 yo F- Still Disease /MAS • Age 19 y fever, rash, cytopenia, pancreatitis, liver disease- intermittent for 4-5 months then persistent • 1 st episode self resolve • 2 nd episode,1 month later: steroids, anakinra • 3 rd episode, 2 months later (prolonged hospital stay): add jakinib (tofacitinib), stopped anakinra before d/c • Weaning tofacitinib after 2 years slowly (BID->QD->QOD) successful so far • IL-18/CXCL9/ferritin – 318000/5000/30000 – 48000/360/1900 – 7000/1200/800 6
- 7. • Age 3 y • 7 mo: fever, rash • ESR:70, CRP:15, high WBC, plt • Anakinra • Wean over 18m (QOD, Q3d->stop) • IL-18/CXCL9/ferritin – 12000/600/600 7 Patient 4 & 5 Google images • Age 5 y • 20 mo: fever, rash, anemia, low plt • ESR 13-35, CRP 2-7 • Steroids (2 ½ m),anakinra (daily for 5 m) • Wean (QOD/4m->Q3d/2m ->stop) • IL-18/CXCL9/ferritin – 250000/600/1600 Google images
- 8. Patient 6 • Age 1 • Started on anakinra (1year) • flare 18 m later; brief anakinra -> canakinumab (weaned off in 1 y) • MAS flare after 3 years (3 days pulse steroids, 1 dose of canakinumab & anakinra BID -> in 1 month to QD • Switched to canakinumab after 2 months • No flares since • All labs normalized (IL-18 fluctuates 700-27000) 8
- 9. GAPS ❖ Need to improve interpretation of genetic test results ❖ Need to understand influence of epigenetics better ❖ Need to solve decreasing drug efficacy issue 9 Google images Google images
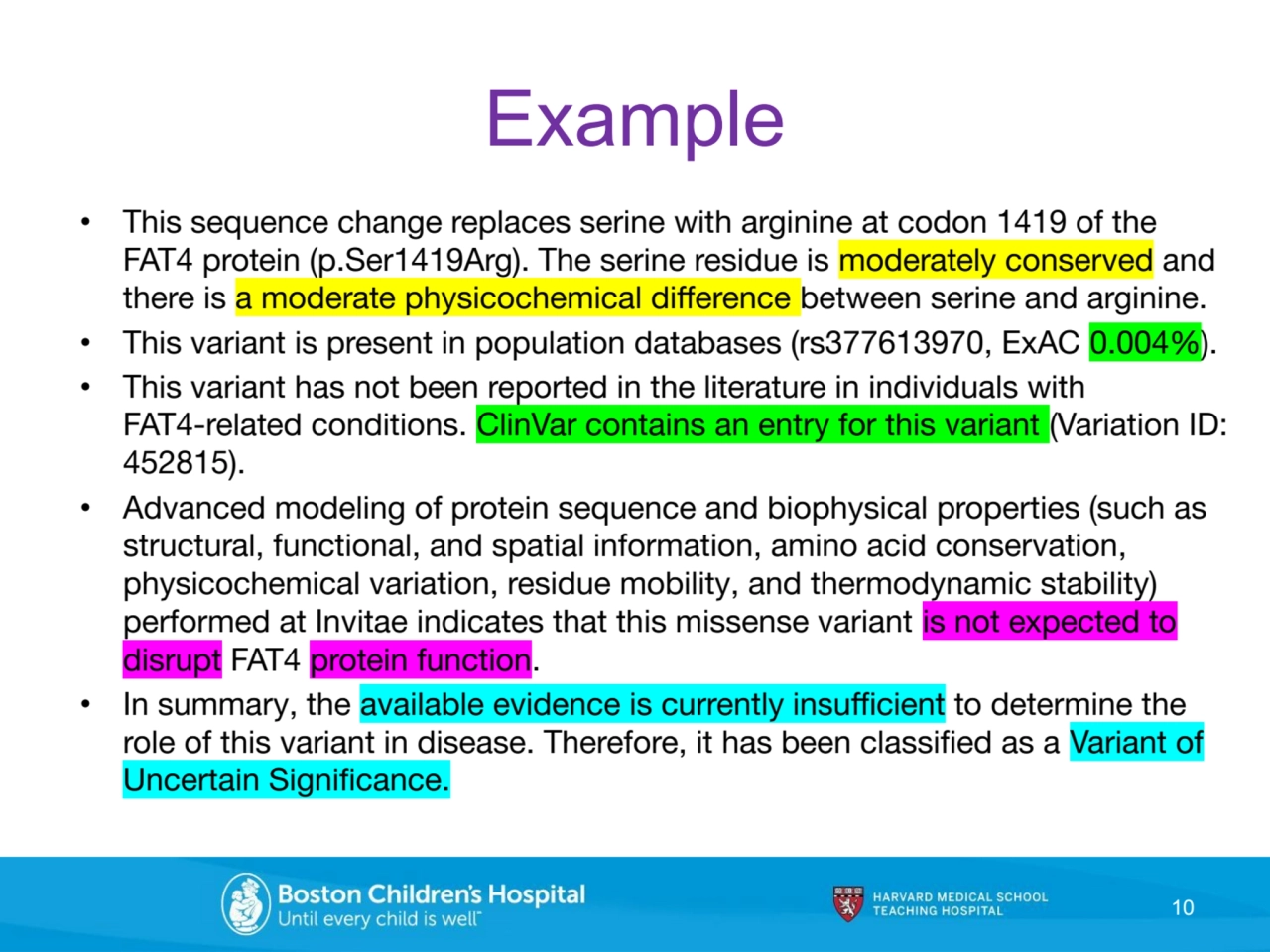
- 10. Example • This sequence change replaces serine with arginine at codon 1419 of the FAT4 protein (p.Ser1419Arg). The serine residue is moderately conserved and there is a moderate physicochemical difference between serine and arginine. • This variant is present in population databases (rs377613970, ExAC 0.004%). • This variant has not been reported in the literature in individuals with FAT4-related conditions. ClinVar contains an entry for this variant (Variation ID: 452815). • Advanced modeling of protein sequence and biophysical properties (such as structural, functional, and spatial information, amino acid conservation, physicochemical variation, residue mobility, and thermodynamic stability) performed at Invitae indicates that this missense variant is not expected to disrupt FAT4 protein function. • In summary, the available evidence is currently insufficient to determine the role of this variant in disease. Therefore, it has been classified as a Variant of Uncertain Significance. 10
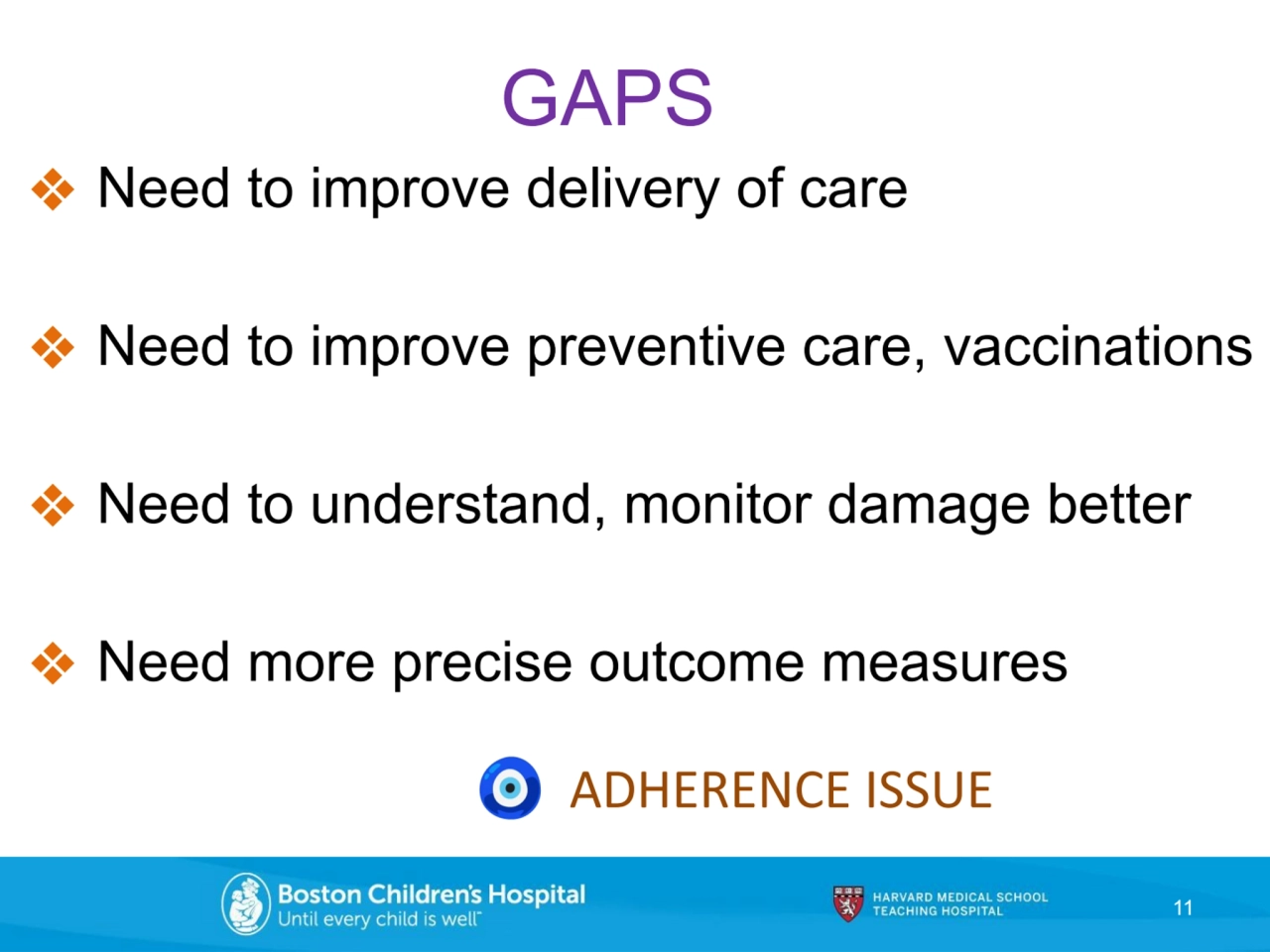
- 11. ❖ Need to improve delivery of care ❖ Need to improve preventive care, vaccinations ❖ Need to understand, monitor damage better ❖ Need more precise outcome measures 11 GAPS ADHERENCE ISSUE
- 12. Follow clues Clinical (similarities to other diseases, patient you see, medication response) Genetic and biologic (does it fit?) 12 Labs (usual, unusual, unique) Summary
- 13. 13 MANAGEMENT MULTIDISCIPLINARY CARE RAPID DISEASE CONTROL PREVENTION OF DAMAGE FROM DISEASE AND TREATMENT PARTICIPATION IN DAILY ACTIVITIES IMPROVEMENT IN HEALTH RELATED QUALITY OF LIFE SHARED DECISION MAKING
- 14. The Key Collaboration 14 Google images
- 15. Boston Children’s Hospital (BCH) Rheumatology Program and Autoinflammatory Clinic staff THANK YOU Our patients and families Lauren Henderson Holly Wobma Pui Lee Peter Nigrovic